Abstract
Background: Ibrutinib is a tyrosine kinase inhibitor (TKI) known to be effective in treating multiple hematologic malignancies. Although generally well tolerated, significant cardiac toxicities including atrial fibrillation, sudden cardiac death, and hypertension may occur shortly after starting therapy with ibrutinib. We aimed to identify predictors of ibrutinib cardiotoxicity in our cohort of patients with non-Hodgkin's lymphoma (NHL) in Northern California.
Methods: We identified 550 adults with NHL who had been newly prescribed ibrutinib between January 2015 through June 2021 and were >60 years at time of first prescription within the Kaiser Permanente Northern California network. Patients with abnormal baseline measures were identified by abnormal echocardiogram (TTE), electrocardiogram (EKG), or blood pressure in the year prior to first ibrutinib prescription. New adverse clinical outcomes, including atrial fibrillation and cardiac and major bleeding events, were identified through diagnosis codes following first ibrutinib prescription. Bivariate comparisons were calculated using Chi-square tests for categorical variables, Student's T-tests for normally distributed continuous variables, and Wilcoxon-Mann-Whitney non-parametric tests for non-normally distributed continuous variables. Kaplan-Meier curves were created for significant clinical outcomes. Analyses were performed on the full NHL cohort and a sub cohort of chronic lymphocytic leukemia (CLL) patients.
Results: Of the 550 patients on ibrutinib, 73.6% carried a diagnosis of CLL, 40% were female, 71.6% were over 70 years of age, and 76.4% identified as White. With respect to comorbid disease, 58.3% were either overweight or obese, 24.9% had diabetes, 48.5% had hypertension, 48.7% had dyslipidemia, 15.3% had liver disease, 31.3% had renal disease, 47% were smokers and 74% reported alcohol use.
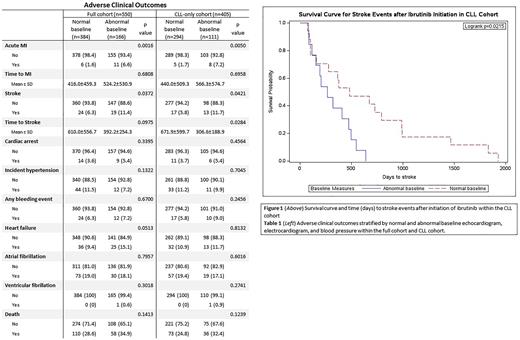
Within the entire cohort, abnormal baseline was associated with higher BMI and hypertension. Patients with abnormal baseline were more likely to have myocardial infarction (MI, p=0.0016) and stroke (p=0.0372). Within the CLL cohort, patients with abnormal baseline were more likely to have MI (p=0.0005) and stroke (p=0.0421). Abnormal baseline was significantly associated with faster time to stroke in the CLL cohort (p=0.0215), but not the full cohort. Interestingly, abnormal baseline was not predictive of atrial fibrillation or major bleeding events and was not associated with patient characteristics such as age, sex, ethnicity, cancer stage, diabetes, liver disease, or kidney disease.
Conclusions: In this cohort, abnormal baseline TTE, EKG and blood pressure measures in patients were associated with MI and stroke, but these factors were not associated with an increase in atrial fibrillation or major bleeding events. The time to stroke was also found to be significantly shorter in CLL patients when compared to the full cohort. Based on these findings, we would recommend cardiac studies prior to initiation of ibrutinib to help define patients at higher risk of adverse cardiovascular events and faster time to stroke.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal